
- Periodic table of elements chemistry reference table pdf#
- Periodic table of elements chemistry reference table series#
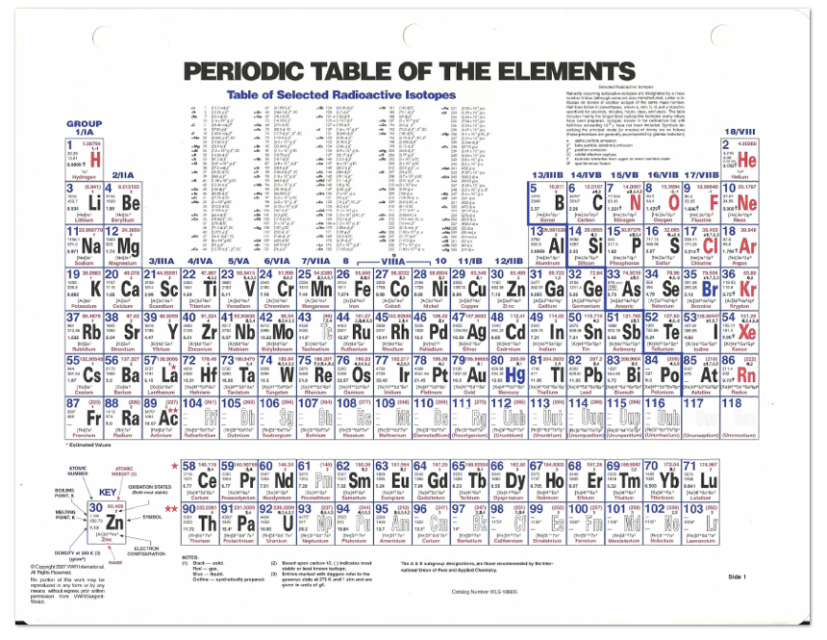
Since its discovery in 1869, the periodic table has guided chemical research including the discovery of new elements. The discovery that elements could be arranged in a periodic table was made by Russian chemist Dmitri Ivanovitch Mendeleev (1834-1907).
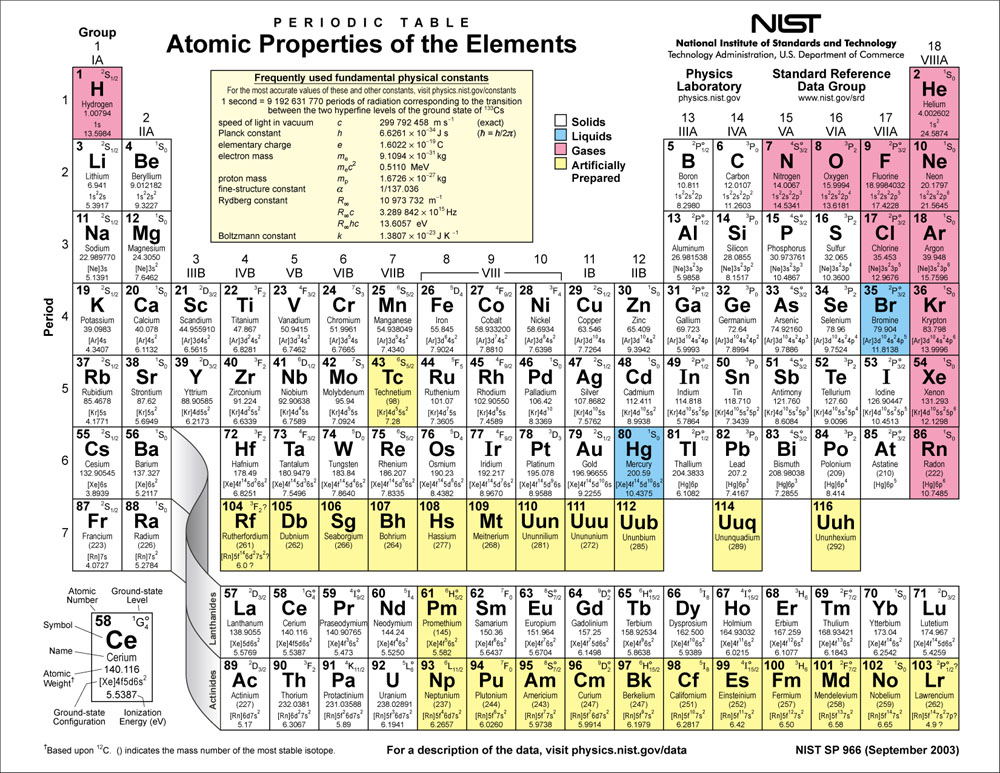
The ultimate effectiveness of the periodic table is that it arranges over one hundred individual elements so information about a given element is known merely by where it is found in the periodic table. Elements of the same group are found to have similar chemical properties. The elements are arranged in order of increasing atomic numbers. Below the element symbol appears the atomic mass, which is the average mass of all the isotopes of that element. These whole numbers are the number of protons present in the nucleus of that element. Above each chemical symbol appears the atomic number of the element. The elements in the table are represented by symbols (one, two, or three letters) in individual squares. Using such rules is not the same as understanding why elements in certain areas the periodic table behave as they do, but the trends that arise from the arrangement of elements in the periodic table allows a chemist to remember useful facts about the types of compounds formed from specific elements and their chemical reactions. From the way elements are organized in the periodic table, chemists can predict their behavior and write chemical formulas of compounds using just a few general guidelines. It has much in common with a thesaurus, providing a guide to similarities and differences among the elements. The periodic table is an essential part of the language of chemistry. Understanding the chemical bond involving heavy and superheavy atoms.The arrangement of the chemical elements into periods (horizontal rows) and groups (vertical columns) is called the periodic table. Relativistic effects, including spin-orbit coupling, are crucial for The donation and back-donation components in coordination bonds, even when Method provides a clear measure (also in combination with the CD analysis) of Likely to play an increasingly important role as one moves down the group.

(CO)$_5$TM-C$_2$H$_4$, with TM =Cr, Mo, W, Sg, where relativistic effects are
Periodic table of elements chemistry reference table series#
Metal-ethylene coordination bond in the group 6-element series As an illustrative example we analyse the In the ADF modelling suite (using the non-relativistic Hamiltonian and the Its correctness and numerical stability have beenĭemonstrated in the case of simple molecular systems, where the relativisticĮffects play a negligible role, by comparison with the implementation available Relativistic four-component Dirac-Kohn-Sham theory that variationally accountsįor spin-orbit coupling. In this work, we extend the EDA-NOCV method to the Scalar level, so simply neglecting the spin-orbit coupling effects and de facto Implementations of the EDA-NOCV scheme include relativistic effects only at The approach has been applied in a variety of chemical contexts, the current Method) in combination with Natural Orbitals for Chemical Valence (EDA-NOCV) isĪ very powerful tool for the analysis of the chemical bonds based on a chargeĪnd energy decomposition scheme within a common theoretical framework. Among others, theĮnergy Decomposition Analysis (EDA, also known as the Extended Transition State The structure, stability and reactivity of chemical species.
Periodic table of elements chemistry reference table pdf#
Download a PDF of the paper titled Chemical bond analysis for the entire periodic table: Energy Decomposition and Natural Orbitals for Chemical Valence in the Four-Component Relativistic Framework, by Diego Sorbelli and Paola Belanzoni and Loriano Storchi and Olivia Bizzarri and Beatrice Bizzarri and Edoardo Mosconi and Leonardo Belpassi Download PDF Abstract: Chemical bonding is a ubiquitous concept in chemistry and it provides aĬommon basis for experimental and theoretical chemists to explain and predict


 0 kommentar(er)
0 kommentar(er)
